Alectinib: A Game-Changer Beyond Lung Cancer – What’s Next?
- moshemelamed6
- Mar 19, 2025
- 3 min read

Alectinib, sold under the brand name Alecensa, is a powerful targeted therapy initially approved for treating ALK-positive non-small cell lung cancer (NSCLC). Since the groundbreaking ALINA trial in 2023, its role has expanded dramatically, showing promise in other ALK-driven cancers.
This article breaks down the latest breakthroughs, ongoing clinical trials, and how patients can determine if Alectinib is the right treatment.
The ALINA Trial: Hope for Early-Stage ALK-Positive NSCLC Patients
The Phase III ALINA trial was a game-changer, testing Alectinib as an adjuvant (post-surgery) treatment for early-stage ALK-positive NSCLC (stages IB-IIIA). Previously, patients relied on chemotherapy, but recurrence rates remained stubbornly high. ALINA set out to change that.
Key Takeaways:
Massive Risk Reduction: Alectinib cut the risk of recurrence or death by 76% compared to chemotherapy.
Stronger Protection Against Brain Metastases: Alectinib significantly lowered the risk of cancer spreading to the brain—a common issue for ALK-positive patients.
Better Tolerability: Fewer patients had to stop treatment due to severe side effects compared to chemotherapy. Common side effects include fatigue, constipation, and liver enzyme changes, though these are often manageable with medical support.
These results led to FDA approval in April 2024, making alectinib the new standard for preventing relapse in surgically removed (resected) ALK-positive NSCLC.
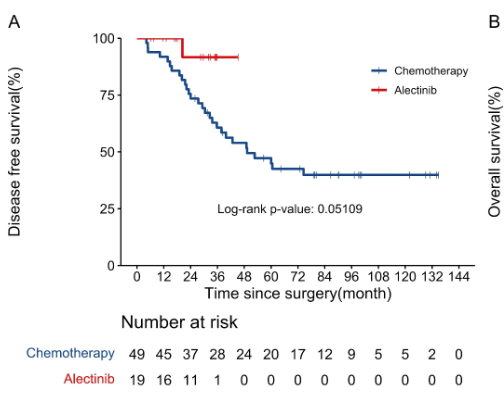
Beyond NSCLC: Alectinib for Patients with Other ALK-Driven Cancers
While NSCLC remains alectinib’s primary focus, researchers are now testing it in other ALK-driven cancers, where early results are promising.
ALK-Positive Anaplastic Large Cell Lymphoma (ALCL)
What is it? A rare type of non-Hodgkin lymphoma with ALK gene mutations.
Why Alectinib? Other ALK inhibitors have worked well in ALCL, and early trials suggest Alectinib could be a safer, effective option.
ALK-Mutated Neuroblastoma
What is it? A pediatric cancer affects the nervous system, with some cases driven by ALK mutations.
Why Alectinib? Preclinical research shows that Alectinib can stop ALK-driven neuroblastoma growth, and trials are now underway for relapsed cases.
Inflammatory Myofibroblastic Tumors (IMTs)
What is it? A rare, aggressive tumor often driven by ALK gene fusions.
Why Alectinib? Case reports show tumor shrinkage in some IMT patients, and trials are investigating its broader potential.
Clinical Trials: New Options for Lung Cancer, Lymphoma, Neuroblastoma, and IMT Patients
Since 2023, several new clinical trials have been launched to explore Alectinib’s potential across different cancers.
For ALK-Positive Lung Cancer (NSCLC):
NAUTIKA1 Trial: Testing alectinib before surgery (neoadjuvant) to shrink tumors and improve surgical outcomes in ALK-positive NSCLC patients.
ALNEO Trial: Similar to NAUTIKA1, evaluating how pre-surgery alectinib affects tumor size and long-term survival in ALK-positive NSCLC patients.
HORIZON-01 Trial: Investigating whether alectinib can prevent post-surgical recurrence better than chemotherapy in ALK-positive NSCLC patients.
For Other ALK-Driven Cancers (Lymphoma, Neuroblastoma, IMTs):
ALKALINE Trial: Studying alectinib in ALK-positive pediatric cancers, specifically neuroblastoma, to see if it can help children with relapsed disease.
IMT-ALK Trial: Evaluating alectinib’s ability to shrink ALK-positive inflammatory myofibroblastic tumors in patients with this rare cancer.
The Future of Alectinib: What’s Next for ALK-Driven Cancer Patients?
Alectinib has already reshaped ALK-positive lung cancer treatment, but its future looks even more promising. With earlier treatment opportunities, expanding cancer types, and ongoing research, more patients than ever may benefit from this breakthrough drug.
If you’re wondering whether Alectinib is right for you, genetic testing and expert opinions are crucial next steps. Connecting with leading cancer centers and specialists like Dr. Elisha Fredman, an expert in ALK-driven cancers who can answer patient questions about clinical trials, is a great place to start. With new trials actively recruiting, now is the time to explore how Alectinib can offer hope for ALK-driven cancer patients worldwide.
Questions or corrections? Reach out to Moshe Melamed at melamed.moshemmd@gmail.com.





Comments